- Visibility 303 Views
- Downloads 49 Downloads
- Permissions
- DOI 10.18231/j.ijor.2023.003
-
CrossMark
- Citation
Management of periarthritis shoulder – Current concept review
Abstract
Adhesive capsulitis, also referred as frozen shoulder or periarthritis shoulder, is a condition marked by shoulder pain, stiffness, and functional restriction of both active and passive shoulder motion. Management of periarthritis shoulder is challenging for orthopaedic surgeons. This article aims at exploring the various treatment modalities for periarthritis shoulder.
Introduction
Adhesive capsulitis, also referred as frozen shoulder or periarthritis shoulder, is a condition marked by shoulder pain, stiffness, and functional restriction of both active and passive shoulder motion.[1] Often it is self-limiting and resolves within 1 to 3 years. It occurs in approximately 2% to 5% of the general population and up to 20% of people with diabetes.[2] The exact pathogenesis of adhesive capsulitis is still unknown. The causes and risk factors of adhesive capsulitis of shoulder is depicted in [Figure 1]. The pathogenesis of adhesive capsulitis is inflammation of shoulder joint capsule and the synovial fluid lining the shoulder joint, which further leads to reactive fibrosis and capsular fibrosis which result in functional loss of shoulder joint movements. Contracture of the glenohumeral capsule is the hallmark of adhesive capsulitis.[3] Diagnosis is based mainly on clinical findings, which include insidious shoulder stiffness, severe pain, especially at night, and loss of both active and passive range of motion.[1]
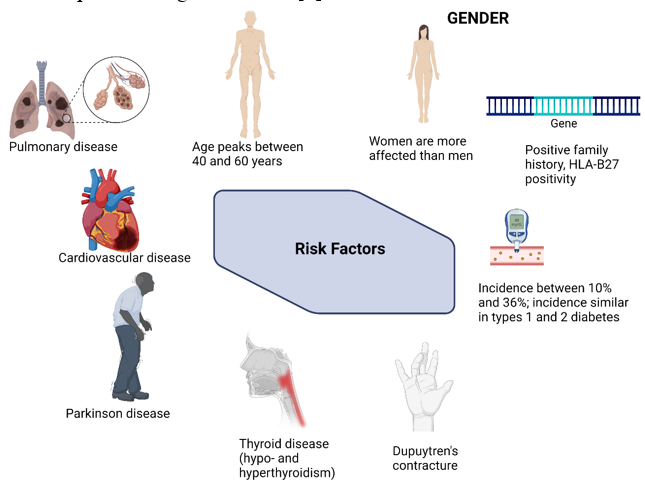
Management of adhesive capsulitis range from conservative treatment to surgical procedures. The treatment options include, alone or often in combination of pharmacologic therapy (including NSAIDs or oral corticosteroids), intraarticular injection, suprascapular nerve block, physical therapy, arthrographic joint distention, or surgical procedures (manipulation under anaesthesia, arthroscopic capsular release, or rarely, open capsulotomy) can be considered for refractory cases. [4] Most patients recover and the disease is often self-limiting, resolving within 1 to 3 years, but the full range of motion may not return.[5] Testing of passive shoulder range of motion is critical in the examination; if testing is omitted, the diagnosis may be missed. True shoulder weakness is not characteristic of adhesive capsulitis; when observed on examination in the absence of pain, other entities should be considered. Participation in postoperative physical therapy is essential for optimal outcomes when surgery is employed.[6]
Stages of Periarthritis Shoulder
Pathological staging
On the basis of clinical presentation and arthroscopic appearance, Neviaser and Neviaser divided the natural progression of adhesive capsulitis into four stages which is depicted in [Figure 2]. [5], [7], [8], [9]
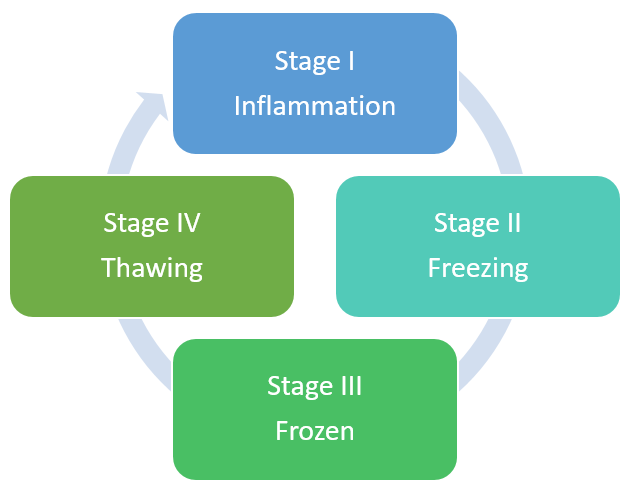
Stage I - Shoulder pain, especially at night, with preserved motion. Arthroscopically, there is evidence of synovitis without adhesions or contractures.
Stage II - Development of stiffness with loss of the axillary fold, suggestive of early adhesion formation and capsular contracture.
Stage III - Profound global loss of ROM and pain at the extremes of motion. Resolving synovitis with obliteration of axillary fold as a result of significant adhesions.
Stage IV - Persistent stiffness but minimal pain as synovitis has resolved. Slow improvement in shoulder mobility is observed. Advanced adhesions and restriction of the glenohumeral joint space was observed arthroscopically.
Histological staging [1], [8]
Stage I - Inflammatory cell infiltration of the synovium
Stage II - Synovial proliferation, and
Stage III - Dense collagenous tissue within the capsule.
MRI staging [10]
|
Stages |
Thickness of anterior band of IGHL |
Edema of IGHL on humeral and glenoid aspect |
Pericapsular edema on anterior and posterior aspect |
Obliteration of fat in the subcoracoid triangle |
|
I |
4.5±0.9 mm |
++ |
++ |
No significant obliteration |
|
II |
7.6±1.9 mm |
++ |
++ |
No significant obliteration |
|
III |
6.4±1.9 mm |
+ |
++ |
Mild obliteration |
|
IV |
5.2±1.3 mm |
- |
_ |
Significant obliteration |
Management of Periarthritis Shoulder
The choice of therapy is based on the stage of the disease, patient's functional status, and individual patient characteristics; treatment is often based on the clinician's personal experience and training. No consensus are found in the current literature on the optimal treatment algorithm. Levels of evidence in studies analyzing treatment outcomes are generally low, especially regarding surgical treatments. Treatment options range from benign neglect to open surgical procedures. Initial treatment is usually conservative. Most patients improve with nonsurgical treatment.
Conservative Treatment Modalities
A retrospective study found that 94% of patients with adhesive capsulitis without treatment recovered to normal levels of function and motion (duration average, 15 months; range, 4-36 months). [11] The disease may be self-limited, but most patients desire more timely intervention and symptom relief. It may have been used in pain management and inflammation; typically used as an adjunct in combination with physical therapy. Pain control is critical in allowing patients to tolerate physical therapy and range of motion exercises
NSAIDs – Generally recommended for short-term pain relief (analgesia) during early inflammatory stages. Not shown to have any effect on the natural course of the disease when used alone.
Oral corticosteroids – Provide significant short-term benefits in shoulder pain, range of motion, and function, but effects may not be maintained beyond 6 weeks.
Intraarticular injection – Typically administered by an orthopedist or non-orthopedic sports medicine physician. Techniques and number of injections are variable. Site of injection (intraarticular versus subacromial) showed no difference in outcome. Accuracy of injection is improved with use of ultrasonography or fluoroscopy versus blind technique.
Corticosteroid intraarticular injection – Thought to inhibit inflammatory phase and reduce pain and stiffness. Effective in short-term pain relief; improved range of motion (short- and long-term), benefitting functional recovery. Provides faster pain relief and earlier shoulder function improvement compared with oral NSAID and oral steroid treatment. Can have deleterious effects on tendon metabolism and articular cartilage.
Sodium hyaluronate intraarticular injection – Unbranched polysaccharide thought to be chondro-protective. Provides equivalent outcomes to intraarticular corticosteroid injections; good safety profile.
Suprascapular nerve block – Block nerves to glenohumeral joint to provide temporary pain relief and facilitate mobilization. Effective in pain relief, but no improvement in shoulder range of motion at 1 month.
Physical therapy – Widely used in conservative management of frozen shoulder. Used to prevent capsular contraction and regain range of motion as symptoms allow. Therapies may include applications of heat or ice, active and passive range-of-motion exercises, mobilization techniques, stretching, patient education, and supervised home exercises. Consider the patient’s symptoms, stage of condition, and patterns of motion loss when selecting physical treatment. Home self-exercise has been found to be as effective as supervised stretching exercise. Discussions concerning the effect, timing, and intensity persist in the literature; in general:
Often used during pain-free phase (frozen
Gentle physical therapy is more effective than intensive therapy
Exercise intensity and range of motion are increased during thawing phase to achieve optimum functionality
Generally combined with other treatment modalities (eg, steroid injection, joint distention
Arthrographic joint distention (brisement) – Injection of fluid (typically saline and steroids) under fluoroscopy and local anesthetic to stretch contracted capsule and increase intracapsular volume. May provide short-term benefits in pain relief, range of motion, and function in adhesive capsulitis. Literature evidence stated that arthrographic joint distention may alleviate pain at 3 weeks and disability up to 12 weeks, but outcome may be no different compared with steroid injection alone.
Surgical Management Options
For persistent symptoms and functional disability refractory to conservative treatment for adhesive capsulitis, surgical intervention is considered. Surgical treatment remains controversial, as condition is typically self-limited. Owing to prolonged pain and disability, patients may elect operative intervention to expedite recovery process, although long-term outcomes may be equivalent. While no discrete timeline has been established for surgical intervention, failed trial of 3 to 6 months of nonoperative care has been suggested. The surgical management options were tabulated in [Table 2].
|
Surgical options |
Significance |
|
Closed manipulation under anaesthesia [12] |
• Mobilization of shoulder beyond normal pain threshold in controlled setting to tear adhesions and stretch contracted capsule; produces capsular rupture leading to improved motion |
|
• Safety and effectiveness remain controversial |
|
|
• Complications can include fractures, dislocation, rotator cuff and labral tears, and neurovascular injuries |
|
|
Arthroscopic capsular release [13] |
• Tightened structures (eg, coracohumeral ligament, rotator interval with contracted capsule) can be released with arthroscopic instruments |
|
• Effective and safe treatment option for recalcitrant adhesive capsulitis |
|
|
• Allows direct visualization to ensure adequate release |
|
|
• Allows confirmation of diagnosis and can rule out other potential causes |
|
|
• Physical therapy and exercise can begin immediately after surgery |
|
|
Open capsulotomy [1] |
• Rarely used for recalcitrant adhesive capsulitis • Remains an option when manipulation under anesthesia and arthroscopic capsular release have failed • Associated with prolonged recovery, stiffness, and postoperative pain that can delay early mobilization |
Recent Advances
Platelet-rich plasma
Platelet-rich plasma (PRP) is defined as the volume of plasma with increased platelet concentration 4 to 5 times above the baseline.[14] The alpha granules present in the platelets contain cytokines, pro- and anti-angiogenic factors, and growth factors [transforming growth factor-beta (TGF-β), basic fibroblast growth factor (b-FGF), epidermal growth factor (EGF), vascular endothelium growth factor (VEGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF), and platelet-derived growth factor (PDGF)].[15] Monocyte chemoattractant, RANTES/CCL5 upon activation, regulates the leucocyte recruitment and attenuates the inflammatory and nociceptive responses.[16] It suppresses the concentration of lipoxin A4 (anti-inflammatory marker), which further depress the number of inflammatory cells. [16] HGF contributes to anti-inflammatory effect by inhibiting NF-κB.[17]
Feusi et al. demonstrated that PRP as a prophylactic measure for secondary adhesive capsulitis of shoulder by reducing the histological grading without causing any adverse effects.[18] In 6 months follow-up, Lin et al observed the improved efficacy of PRP than procaine in the management of frozen shoulder. [19] Serdar et al reported no improvement in functional quality of life and shoulder range of movements by PRP than placebo in patients with rotator cuff tendinopathy at 1-year follow-up.[20] Muthu et al reported improved healing rate at the augmentation of PRP at bone-tendon interface, reducing re-tear rates, and mitigate pain in patients with rotator cuff tears (RCTs). [21] Lee et al demonstrated decreased pro-inflammatory cytokines in the inflammatory phase of adhesive capsulitis when treated with allogenic pure PRP. Allogenic PRP reported no side effects. [22] PRP improved IL-1β−induced synovial inflammatory condition by downregulating proinflammatory cytokines such as IL-1β, TNF−α, IL-6, COX-2, and microsomal PGE2 synthase−1 and decreased MMP−1, −3, and −13, disintegrin and metalloproteinase with thrombospondin motifs−4 and −5 and upregulating anti-inflammatory cytokines such as vasoactive intestinal peptide.[23], [24] Xu et al reported reduced long term re-tear rate and improved quality of life in patients with RCT treated with PRP. [25] PRP promote healing of small to medium-sized tears of RCT to reduce re-tear rates whereas in large tears, double-row repair with PRP augmentation reduce re-tear than PRP augmentation alone. [26]
Mesenchymal stromal cells (MSCs and exosomes)
International Society for Cellular Therapy (ISCT) has laid down the minimal criteria for clinical and translational usage of MSCs. [27] Tendon environment hinders the native regenerative process, which will impede the administered MSCs. Tendon differentiation from injected MSCs is achieved through either peppering technique or augmentation with biological substances and single or multiple injections.
The completed animal trials on MSC for rotator cuff disorders revealed a significantly reduced number of re-tears at 10 year follow-up, improved tendon integrity as evidenced by MRI analysis, and reduced rate of adverse reactions. [28] Muthu et al emphasized the utilization of cellular therapy in RCT with reduced pain, improved ROM at 3 and 6 months, and enhanced tendon healing. [29] Kim et al demonstrated the excellent outcome with the combination of BMAC-PRP in partial tear RCT. [30] In vivo rat model of RCT, freshly thawed UC-MSCs regenerate full thickness rotator tendon defect. UC-MSCs attenuates inflammation, reduces RCT defect size, and improves slidability by 2 weeks. Histologically, fiber coherence, organization of collagen fibrils, fibroblast orientation in the tendon tissue were improved with UC-MSCs in RCT in rat model. [31]
Transplantation of BM-MSC derived Exos facilitates angiogenesis by boosting the release of VEGF and attenuates inflammatory response by secreting anti-inflammatory factors by M2 macrophages which helps in rotator cuff tendon repair.[32], [33] Human BM-MSC-derived EVs inhibit shoulder stiffness via let-7a/TGFBR-1 axis in patients with frozen shoulder. [34] BM-MSC derived EVs demonstrated the compactly aligned collagen fibers when administered intra-tendinously. BM-MSC derived EV group showed a significantly higher concentration of COL-1A1, scleraxis, and tenomodulin, and anti-inflammatory mediators after 2 weeks of surgery.
Zhang et al demonstrated the tendoprotective and anti-inflammatory effects of AD-MSC derived Exos with concomitant use of glucocorticoids (GC) for rotator cuff tendon pathology.35 AD-MSC derived Exos antagonise the detrimental effects of GC on rotator cuff tendons by improving GC suppressed cellular proliferation, migration and transcription of tenocytic matrix molecules. In vivo studies revealed that AD-MSC derived Exos restored histological and biomechanical function of rotator cuff tendons. [35]
Whole body cryotherapy
As a result of technological developments, cryotherapy has emerged as a popular option for the anaesthesia of orthopaedic injuries. Whole-body cryotherapy, also known as WBC, is a treatment that involves exposing the patient to extremely cold air for a short period of time while having them wear only the barest minimum of clothing. temperature-controlled chamber keeps the air at a constant temperature of between 110 and 140 degrees Celsius.[36]
Patients with fibromyalgia, rheumatoid arthritis, chronic low back pain, osteoarthritis, and ankylosing spondylitis who had a temperature of 100°C WBC in the 1990s were found to experience a sudden reduction in their skin temperature when they were given WBC. After being exposed to WBC, researchers found that there were anti-inflammatory and analgesic effects on the blood's balance of antioxidants and prooxidants. It is believed that the slowing of nerve conduction was caused by an increase in both the concentration and concentration of endorphin in the afferent fibres, which are responsible for receiving pain signals [36]
Patients diagnosed with AC receive treatment for the condition using thermal and electrical modalities, with the goals of reducing pain and improving physical function. More recently, these treatments have been viewed as an adjunct to the management of adhesive capsulitis.[36], [37]
Future Directives
Enzymatic capsulotomies
Enzymatic capsulotomies adapt an enzyme that breaks down collagen's peptide bonds that is produced by Clostridium histiolyticum. Collagenase has been approved by the FDA to treat Dupuytren's disease and Peyronie's disease, two fibrotic tissue disorders (FDA). Many aspects of Dupuytren's disease and adhesive capsulitis are similar, including histological and molecular similarities. Patients with adhesive shoulder capsulitis have been able to receive FDA-approved collagenase injections off-label for use in Dupuytren's disease only recently.
Botox injection
Botulinum toxin is produced by the bacteria Clostridium botulinum. Botulinum neurotoxins are currently being used in the treatment of spastic disorders associated with stroke, multiple sclerosis (Parkinson's), Parkinson's disease, and cerebral palsy, as well as migraine therapy, cervical dystonia, and chronic musculoskeletal pain.
Conclusions
Management of adhesive capsulitis pose a greater challenge among orthopaedic surgeons. The usage of appropriate modality of treatment may decrease the morbidity and improve the functional quality of life of the affected individuals.
Conflict of Interest
None.
Source of Funding
None.
References
- Le H, Lee S, Nazarian A, Rodriguez E. Adhesive capsulitis of the shoulder: review of pathophysiology and current clinical treatments. Shoulder Elbow. 2017;9(2):75-84. [Google Scholar] [Crossref]
- Uppal H, Evans J, Smith C. Frozen shoulder: A systematic review of therapeutic options. World J Orthop. 2015;6(2):263-8. [Google Scholar]
- Patel R, Urits I, Wolf J. A Comprehensive Update of Adhesive Capsulitis and Minimally Invasive Treatment Options. Psychopharmacol Bull. 2020;50(4):91-107. [Google Scholar]
- Jeyaraman M, Ramesh R, GP, Dhamsania H. The comparative and prospective study on efficacy and functional outcome of autologous platelet rich plasma injection vs hydrodissection in adhesive capsulitis of shoulder. Int J Res Orthop. 2018;4(6):848-53. [Google Scholar] [Crossref]
- Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ. 2005;331(7530):1453-6. [Google Scholar]
- Hoogeboom T, Dronkers J, Hulzebos E, Meeteren NV. Merits of exercise therapy before and after major surgery. Curr Opin Anaesthesiol. 2014;27(2):161-6. [Google Scholar]
- Chan H, Pua P, How C. Physical therapy in the management of frozen shoulder. Singapore Med J. 2017;58(12):685-9. [Google Scholar]
- Neviaser A, Neviaser R. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 2011;19(9):536-42. [Google Scholar]
- Neviaser J, Neviaser R. Adhesive capsulitis of the shoulder. J Am Acad Orthop Surg. 1945;27(2):211-22. [Google Scholar]
- Chellathurai A, Subbiah K, Elangovan A, Kannappan S. Adhesive capsulitis: MRI correlation with clinical stages and proposal of MRI staging. Indian J Radiol Imaging. 2019;29(1):19-24. [Google Scholar]
- Vastamäki H, Kettunen J, Vastamäki M. The natural history of idiopathic frozen shoulder: a 2- to 27-year followup study. Clin Orthop Relat Res. 2012;470(4):1133-43. [Google Scholar]
- Kraal T, Beimers L, The B, Sierevelt I, Bekerom MVD, Eygendaal D. Manipulation under anaesthesia for frozen shoulders: outdated technique or well-established quick fix?. EFORT Open Rev. 2019;4(3):98-109. [Google Scholar] [Crossref]
- Arce G. Primary Frozen Shoulder Syndrome: Arthroscopic Capsular Release. Arthrosc Tech. 2015;4(6):e717-20. [Google Scholar] [Crossref]
- Alves R, Grimalt R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Skin Appendage Disord. 2018;4(1):18-24. [Google Scholar] [Crossref]
- Blair P, Flaumenhaft R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009;23(4):177-89. [Google Scholar] [Crossref]
- Ameer LA, Raheem Z, Abdulrazaq S, BA, MN, AK. The anti-inflammatory effect of the platelet-rich plasma in the periodontal pocket. Eur J Dent. 2018;12(4):528-31. [Google Scholar] [Crossref]
- Bendinelli P, Matteucci E, Dogliotti G. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225(3):757-66. [Google Scholar]
- Feusi O, Karol A, Fleischmann T, Rechenberg B, Bouaicha S, CW. Platelet-rich plasma as a potential prophylactic measure against frozen shoulder in an in vivo shoulder contracture model. Arch Orthop Trauma Surg. 2022;142(3):363-72. [Google Scholar] [Crossref]
- Lin J. Platelet-rich plasma injection in the treatment of frozen shoulder: A randomized controlled trial with 6-month follow-up. Int J Clin Pharmacol Ther. 2018;56(8):366-71. [Google Scholar] [Crossref]
- Kesikburun S, Tan A, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41(11):2609-16. [Google Scholar] [Crossref]
- Muthu S, Jeyaraman N, Patel K. Evidence analysis on the utilization of platelet-rich plasma as an adjuvant in the repair of rotator cuff tears. World J Meta-Analysis. 2022;10(3):143-61. [Google Scholar]
- Lee M, Yoon K, Oh S, Shin S, Jo C. Allogenic Pure Platelet-Rich Plasma Therapy for Adhesive Capsulitis: A Bed-to-Bench Study With Propensity Score Matching Using a Corticosteroid Control Group. Am J Sports Med. 2021;49(9):2309-20. [Google Scholar] [Crossref]
- Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents. 2012;26(2):35-42. [Google Scholar]
- Mariani E, Roffi A, Cattini L. Release kinetic of pro- and anti-inflammatory biomolecules from platelet-rich plasma and functional study on osteoarthritis synovial fibroblasts. Cytotherapy. 2020;22(7):344-53. [Google Scholar] [Crossref]
- Xu W, Xue Q. Application of Platelet-Rich Plasma in Arthroscopic Rotator Cuff Repair: A Systematic Review and Meta-analysis. Orthop J Sports Med. 2021;9(7). [Google Scholar] [Crossref]
- Vavken P, Sadoghi P, Palmer M. Platelet-Rich Plasma Reduces Retear Rates After Arthroscopic Repair of Small- and Medium-Sized Rotator Cuff Tears but Is Not Cost-Effective. Am J Sports Med. 2015;43(12):3071-6. [Google Scholar]
- Dominici M, Blanc KL, Mueller I, . Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-17. [Google Scholar] [Crossref]
- Morton-Gonzaba N, Carlisle D, Emukah C, Chorath K, Moreira∗ A. Mesenchymal stem cells and their application to rotator cuff pathology: A meta-analysis of pre-clinical studies. Osteoarthr Cartil Open. 2020;2(2). [Google Scholar] [Crossref]
- Muthu S, Mogulesh C, Viswanathan VK. Is cellular therapy beneficial in management of rotator cuff tears? Meta-analysis of comparative clinical studies. World J Meta-Analysis. 2022;10(3):162-76. [Google Scholar]
- Kim S, Kim E, Kim S, Song D. Effects of bone marrow aspirate concentrate and platelet-rich plasma on patients with partial tear of the rotator cuff tendon. J Orthop Surg Res. 2018;13(1). [Google Scholar] [Crossref]
- Yea J, Park J, Kim I, Sym G, Bae T, Jo C. Regeneration of a full-thickness defect of rotator cuff tendon with freshly thawed umbilical cord-derived mesenchymal stem cells in a rat model. Stem Cell Res Ther. 2020;11(1). [Google Scholar] [Crossref]
- Huang Y, He B, Wang L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11(1). [Google Scholar] [Crossref]
- Shi Z, Wang Q, Jiang D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J Transl Med. 2019;17(1). [Google Scholar] [Crossref]
- Luo Z, Sun Y, Qi B, JL, Chen Y, Xu Y. Human bone marrow mesenchymal stem cell-derived extracellular vesicles inhibit shoulder stiffness via let-7a/Tgfbr1 axis. Bioact Mater. 2022;17:344-59. [Google Scholar] [Crossref]
- Zhang X, Li A, Han K. Anti-inflammatory and Tendon-Protective Effects of Adipose Stem Cell-Derived Exosomes with Concomitant Use of Glucocorticoids. Stem Cells Int. 2022. [Google Scholar] [Crossref]
- Bleakley C, Bieuzen F, Davison G, Costello J. Whole-body cryotherapy: empirical evidence and theoretical perspectives. Open Access J Sports Med. 2014;5:25-36. [Google Scholar] [Crossref]
- Ma S, Je H, Jeong J, Kim H, Kim H. Effects of whole-body cryotherapy in the management of adhesive capsulitis of the shoulder. Arch Phys Med Rehabil. 2013;94(1):9-16. [Google Scholar]
How to Cite This Article
Vancouver
Kolathuru S, Khanna M, Jayaraman M. Management of periarthritis shoulder – Current concept review [Internet]. IP Int J Orthop Rheumatol. 2023 [cited 2025 Sep 25];9(1):19-24. Available from: https://doi.org/10.18231/j.ijor.2023.003
APA
Kolathuru, S., Khanna, M., Jayaraman, M. (2023). Management of periarthritis shoulder – Current concept review. IP Int J Orthop Rheumatol, 9(1), 19-24. https://doi.org/10.18231/j.ijor.2023.003
MLA
Kolathuru, Sudhaker, Khanna, Manish, Jayaraman, Madhan. "Management of periarthritis shoulder – Current concept review." IP Int J Orthop Rheumatol, vol. 9, no. 1, 2023, pp. 19-24. https://doi.org/10.18231/j.ijor.2023.003
Chicago
Kolathuru, S., Khanna, M., Jayaraman, M.. "Management of periarthritis shoulder – Current concept review." IP Int J Orthop Rheumatol 9, no. 1 (2023): 19-24. https://doi.org/10.18231/j.ijor.2023.003
