- Visibility 60 Views
- Downloads 8 Downloads
- DOI 10.18231/j.ijor.2022.002
-
CrossMark
- Citation
Sources of mesenchymal stem cells and its potential
- Author Details:
-
Abhishek Singh *
-
Manish Khanna
-
Arun Gulati
-
Gautam Sinha
-
Satyajeet Verma
Introduction
Friedenstein in 1976 first described mesenchymal stem cells (MSCs) which were discovered from bone marrow.[1] Since then, interest in these adult MSCs has been progressively increasing. Stem cells posses two characteristic features, they can differentiate along variable cell lineages and can renew themselves. Stem cells can be divided in two major variety, namely, embryonic stem cells and adult stem cells. Embryonic stem cells (ESC) are derived from the blastocyst inner cell mass and are related with tumorigenesis. Usage of human Embryonic Stem cells posses ethical and legal consideration. Mesenchymal stem cells are basically stromal cells. MSCs can be procured from different tissue sources, such as umbilical cord, synovial tissue, breast milk, menstrual blood, adipose tissue, bone marrow, dentine pulp etc.[2] These MSCs have a critical role in tissue injuries, tissue regeneration, organogenesis, gene therapy and organ transplant. These MSCS can be used therapeutically in numerous tissue regenerative and repair activity in musculoskeletal ailments including avascular necrosis of femoral head, osteoarthritis of joints, ligamentous injuries, meniscal injuries, intervertebral disc diseases. Inducing growth in paediatric patients with diseases like osteogenesis imperfecta, muscular dystrophies.
However MSCs isolation from adult tissues is relatively advantageous in contrast to ESCs when they are compared ethically, legally and their immunological privileges. Research work is continuously going on in the field of finding different new sources of MSCS, which will broaden the usage of these cells in different areas of medical field. Recently numerous sources of MSCS have emerged out and being studied for their therapeutic and clinical applications. By this review, attempt has been made to study recent advances in emerging new sources of mesenchymal stem cells and their therapeutic potentials.
Properties and characteristics of mesenchymal stem cells
International Society for Cellular Therapy (ISCT) have proposed minimal criteria to define human MSC. [3] First, MSC are adherent to plastic surfaces when grown in suitable culture conditions. Second, MSC must express CD105, CD73 and CD90 and absence of expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface moleculesas analysed by flow cytometry. Third, MSC are capable to differentiate into osteoblasts, adipocytes and chondroblasts in vitro. Although with new update of knowledge in the field of MSCS, these criteria will also be needed to be modified and upgraded. The main characteristics which differentiates stem cells from rest of cells of the adult body is that, in suitable condition stem cells divide specifically and make their own numerous number of copies, having the same morphology and features. Second, they are capable to differentiate in several different cell lineages including cardiomyocytes, hepatocytes, nephrons, neurons and myocytes.
In short MSCs are functionally active in self-maintenance, multipotent (have the capability to differentiate into more than one cell type) and pleuripotent (have the capacity to differentiate into all three germ layers-ectoderm, mesoderm and endodermal cell derivatives). Hence, stem cells differ in their potential by their source (embryonic, adult etc.).
Immunosuppressive and Immunomodulatory Properties of MSCs
MSCs have immunosuppressive and immunomodulatory features along with differentiation and regenerative properties. Which is crucial to make them efficient in cellular therapy. By virtue of these feature MSCs are used in the treatment of graft-versus-host disease (GVHD). Mesenchymal stem cells are immunoprivileged cells since expression of class II Major Histocompatibilty Complex (MHC-II) and costimulatory complex is low. They also manipulate different immune response pathways by means of direct cell-to-cell interactions. MSCs suppress proliferation of T cells, B-cells, natural killer cells (NK) and dendritic cells (DC) and produce division arrest anergy. Beside this, MSCs can alter cytokine and antibody production, cytotoxicity of T cells, B cells and dendritic cell maturation and activation as well as antigen presentation. [4] These characteristics were first utilised in hematopoietic stem cells transplantation to treat drug resistant graft-versus-host disease and very effective in organ transplantation by lowering the incidence of rejection.[5]
Sources and their potentials
Tissue source determines the differentiation potentials of mesenchymal stem cells.[6] Embryonic Stem Cells (ESCs) can proliferate and differentiate into all the three germ layers derivatives (ectoderm, endoderm and mesoderm) in vitro. Hence they have been used in regenerative medicine and treatment of genetic disorders. However, embryonic stem cells are immunogenic when they grow and differentiate in vivo. In contrast, adult mesenchymal stem cells, derived from bone marrow source are found to be multipotent and can differentiate into cells of mesodermal (osteocytes, chondrocytes, and adipocytes), ectodermal (neurons) and endodermal origins. Other eminent and newer sources of adult MSCs include:
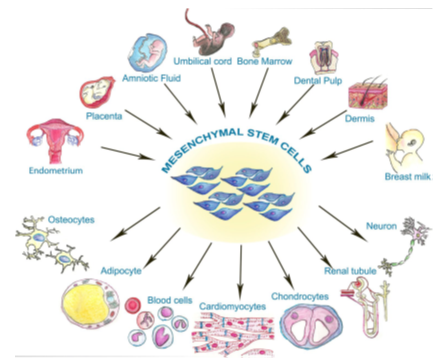
Umbilical cord derived MSCs (UC-MSCs)
Umbilical cord has a gelatinous connective tissue called wharton’s jelly and contains stem cells which can be harvested for therapeutic uses. Umbilical cord derived MSCs can be used to treat chondral, osteochondral lesions and bone injuries. Umbilical cord derived MSCS are superior to other sources derived MSCs in some perspective. These cells falling between embryonic stem cells and adult mesenchymal stem cells developmentally. They do not give rise to tumorigenesis and are hypoimmunogenic in nature. UC-MSC differentiate into the osteogenic, adipogenic and chondrogenic lineage. These cells also posses immunomodulation and decrease morbidity in transplantation procedures. [7]
Amniotic fluid derived MSCs
Human amniotic fluid have been implicated in diagnosis of congenital fetal disorders and genetic abnormalities. Amniotic fluid has heterogeneous undifferentiated and partially differentiated cells since some cells are also derived from fetus. In various studies so far, it has been shown that amniotic fluid stem cells can differentiate into adipogenic, osteogenic, myogenic, endothelial, neurogenic and hepatic cells lineages. In contrast to embryonic stem cells, amniotic fluid-derived MSCs don’t induce tumorogenesis. In a study performed by Preeti Deedwania, they isolated the MSCs from the amniotic fluid and possesed typical MSC surface markers and were able to differentiate into osteocytes which was confirmed by staining with Alizarin Red S stain, chondrocyte (positive staining with Alcian Blue after 2 weeks of culture) and adipocytes (positive staining with Oil Red O stain after 3 weeks of culture). Amniotic fluid MSCs give better result in context of bone healing in contrast to Bone Marrow derived MSCs when tried in vivo. Hence amniotic fluid derived MSCs can be used in bone healing with better results. [8]
Adipose tissue derived MSCs (ATMSCs)
Human adipose tissue can be easily procured by minimally invasive procedure. Autologous adipose tissue derived MSCs has opened a gateway for both regenerative medicine and tissue injury and diseases and has become progressively expanding area of research9. Adipose tissue derived mesenchymal stem cells (ATMSCs) can differentiate into adipocytes, chondrocytes, osteocyte, myocytes and neurogenic phenotype. These cells act by mineralizing extracellular matrix, increases osteocalcin and alkaline phosphatase expression. These MSCsare also chondrogenic when grown in alginate culture as well as in vivo. However in a study[9] it was shown that osteogenenesis potential of adipose tissue derived MSCs is less than that of the bone marrow derived MSCs which was demonstrated by alkaline phosphatase staining. These two MSCs also differ quantitatively from each other in terms of osteogenesis as demonstrated by Von Kossastaining. However above results are different from the Urgarte et al. observations who has demonstrated that there is no statistically significant difference in osteogenesis between bone marrow derived MSCs and ATMSCs. Although he used different medium for differentiation. It can be supposed that ATMSCs needs more specific medium for an effective osteogenesis both qualitatively as well as quantitatively. Chondrogenesis potential of ATMSCs is also very low.
Dental pulp derived MSCs (DPSCs)
In year 2000, a special type of stem cells were described by Gronthos et al. which were present in human dental pulp. These cells had features of MSCs like adherence to plastic surfaces, lack of expression of CD14, CD34 and CD45. These cells can differentiate into osteoblast as well. Beside this, these cells has similarity with BM-MSCs antigenically and capacity to proliferate is higher in contrast to BM-MSCs. DPSCs are the neural crest derivatives, which has ectodermal origin. Thus, these MSCS have privilege to produce neural cells and regeneration of neural tissue due to their developmental origin, in contrast to other sources of MSCs. Due to this these cells are being studied in detail for their application in treatment of neurodegenerative disorders. Moreover these cells have osteogenic potential too when cultured under suitable conditions. Flat bones which are developed from intramembranous ossification like bones of the skull, mandible, are derived from neural crest stem cells. When DPSCs were identified as MSCs which originate from treatment of jaw defects. Dentine pulp derived MSCs can also be implicated in dentin regeneration. DPSCs have a greater osteogenic differentiation potential and has similarity in potential to differentiation into chondrocytes and adipocytes in contrast to umblical cord derived MSCs. [10]
Breast milk derived MSCs
Breast milk not only contains chemical compounds which are nutritious, provide immunity and impart growth to the infant but cells which meets the criteria for MSCs.[11] In a study conducted by Somia H. Abd Allah,[12] MSCs were extracted from breast milk of rabbits and theses cells has close resemblance with human MSCs (hMSCs). Rabbit serves as easily accessible source of MSCs as compared to other larger size animals. In this study, it was revealed that these MSCs has a positive expression for CD29, CD166, CD44, CD105 and nestin but lack expression of other surface markers as CD45 and CD34 which meets the essential criteria for referring any cell to be as MSCS. Patki et al. [13] succeeded in isolation of MSCs from human breast milk. These cells met criteria for MSCs and has positivity for MSC surface markers CD44, CD29, SCA-1 and lack expression of CD33, CD34, CD45, CD73. These cells also have expression of MSCs markers like nestin, vimentin smooth muscle actin. This variety of MSCs has the potential for differentiation into adipocytes, chondrocytes and osteoblast lineage under suitable conditions and act as good candidate for their use in regenerative therapy.
Synovium derived MSCs
Synovial tissue can be easily procured by arthroscopy and act as precious source of MSCs. In previous comparative studies it has been shown that MSCs derived from synovium, bone marrow and periosteum have more pronounced chondrogenesis potential than adipose tissue derived MSCs or muscle derived MSCs. Amongst these, synovium-derived MSCS have highest chondrogenic potential. They also compared osteogenesis potential of bone marrow, synovium and periosteum derived MSCs by alizarin red positivity and it was found that their osteogenesis potential is more profound than that of adipose tissue and muscle derived MSCs.[14] In adipogenesis studies too, synovium and adipose tissue-derived MSCs shown maximum oil red O positivity.[15]
Peripheral blood derived mesenchymal stem cells (PBMSCs)
PBMSCs can be easily harvested from blood with higher sterillity and less invasive way and act as an efficient source of autologous MSCs for clinical application.[16] Recently interest in PBMSCs has profoundly increased as they have equivalent biological attributes similar to MSCs derived from bone marrow or adipose tissue. Under suitable culture conditions the PBMSCs have the potential to differentiated into osteoblast, chondroblast, adipocyte, neuron.[17] In a study performed by Pan Panchong demonstrate that Peripheral Blood derived MSCs and Bone Marrow derived MSCs have similar chondrogenic differentiation potential, Hence these MSCs derived from peripheral blood can act as a good substitute for chondral repair.[18] In a study for assessing osteogenesis potential of theses peripheral blood derived MSCs demonstrated that these MSCs can undergo multilineage differentiation including osteoblasts both in vitro and in vivo. [19]
Periosteum derived MSCs
Periosteumen encompasses special type of MSC population. Periosteum derived mesenchymal stem cells have the capacity to differentiate into chondrocytes and osteocytes in vitro and in vivo along with adipocytes in vitro and therefore are being used in regenerative therapy of musculoskeletal diseases. These cells posses enormous capacity for self renewal and multilineage differentiation and fulfill the criteria for MSCs as they have cell markers of MSCs. These MSCs are multipotent even at single cell level and can differentiate into chondrocytes, osteocytes, adipocytes and myocytes both in vitro and in vivo.[20] These cells can be very easily harvested by simple biopsy of bone periosteum. This mutipotency property is supposed to be arisen due to multiple different progenitor cells arising from different layers of periosteum like fibrous and cambium layer.
Menstrual fluid derived MSCs
Mesenchymal stem cells/stromal cell population was isolated a little while back from continuously dividing and regenerating human endometrium as clonogenic stromalcells which are highly proliferative in nature and comply the criteria for MSCs according to International Society for Cellular Therapy (ISCT). Embryologically, endometrium is mesodermal derivative. Thus, undoubtedly endometrial MSCs and stromal fibroblasts can differentiate into mesodermal lineages. Differentiation of menstrual fluid derived MSCs into classic mesodermal lineages (osteocytes, chondrocytes, adipocytes) in vitro has been demonstrated. Endometrial stromal fibroblasts have similarity with bone marrow-derived stromal cells for mesodermal lineage differentiation. But collectively, the differentiation capability of unfractionated MSCs which are isolated by using traditional methods have lower potential as compared to bone marrow derived MSCs isolated in a similar manner for differentiation into osteoblast and adipocytes. These differences can be due to different tissue of origin. These menstrual fluid derived MSCs can differentiate into endodermal lineages like hepatocytes in vitro and insulin and glucagon secreting pancreatic lineages both in vitro and in vivo. [21]
Induced pleuripotent MSCs
Human induced pluripotent stem cell-derived mesenchymal stem cells (hiPSC-MSCs) have come out as a new source of stem cells which can be used in regenerative science. Exosomes extracted from this group of stem cells may play significant role in tissue repair. However until now there are no studies which have demonstrated the use of hiPSC-MSC-Exosomes in tissue repair and underlying mechanisms in tissue repair is also poorly understood. [22]
Comparison of potentials of various sources of MSCs
The differences in proliferative, differentiation and immunomodulation potential of different stem cells depends upon the source they are derived from.
|
S.No. |
Mesenchymal stem cells |
Source |
Potential |
|
1. |
Bone marrow derived MSCs |
Iliac crest |
Potential to regenerate bone and cartilage, Auto and allogenicity |
|
2. |
Adipose derived MSCs |
Abdomen, medial aspect of thigh |
Potential to regenerate cartilage and soft tissue. |
|
3. |
Synovium derived MSCs |
Synovium around knee joint |
Potential to regenerate bone and cartilage |
|
4. |
Umbilical cord derived MSCs |
Umblica -cord, whartons jelly |
Pleuripotent in nature. |
|
5. |
Amniotic fluid derived MSCs |
Cytotrop- hoblast, Syncytiotrophoblast |
Pleuripotent in nature. |
|
6. |
Peripheral blood derived MSCs |
Circulating mononuclear cells |
Enhanced osteogenic and adipogenic potentials. |
|
7. |
Dentine pulp derived MSCs |
Teeth |
Pluripotent in nature, osteogenic potential. |
Conclusion
Mesenchymal stem cells have revolutionized the medical science over the time. This continuously growing and propagating field is multidisciplinary and have brought the biologist, clinicians, surgeons, biotech and pharmaceutical industry together. Advancement in knowledge of newer and latest source of stem cells has laid down the platform for immense research in field of stem cells and their therapeutic application in different ailments of human body and has given a new dimension to the regenerative medicine.
Conflict of Interest
The authors hereby declare that there is no conflict of interest..
Source of Funding
None.
References
- R Mafi. Sources of Adult Mesenchymal Stem Cells Applicable for Musculoskeletal Applications - A Systematic Review of the Literature. Open Orthop J 2011. [Google Scholar]
- DC Ding, WC Shyu, SZ Lin. Mesenchymal Stem Cells. Cell Transplantation 2011. [Google Scholar] [Crossref]
- M Dominici, K Le Blanc, I Mueller, I Slaper-Cortenbach, FC Marini, DS Krause. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006. [Google Scholar] [Crossref]
- MP De Miguel, S Fuentes-Julián, A Blázquez-Martínez, CY Pascual, MA Aller, J Arias. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012. [Google Scholar] [Crossref]
- KL Blanc, I Rasmusson, B Sundberg, C Götherström, M Hassan, M Uzunel. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004. [Google Scholar] [Crossref]
- L Xu, Y Liu, Y Sun, B Wang, Y Xiong, W Lin. Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res Ther 2017. [Google Scholar] [Crossref]
- A Marmotti, S Mattia, F Castoldi, A Barbero, L Mangiavini, DE Bonasia. Allogeneic Umbilical Cord-Derived Mesenchymal Stem Cells as a Potential Source for Cartilage and Bone Regeneration: An In Vitro Study. Stem Cells Int 2017. [Google Scholar] [Crossref]
- P Deedwania, D Deka, S Mohanty, V Dadhwal, A Sharma. Isolation and characterization of mesenchymal stem cells derived from amniotic fluid: A prospective study. Int J MolImmuno Oncol 2020. [Google Scholar]
- Gun-Il Im, YW Shin, KB Lee. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells?. Osteoarthritis Cartilage 2005. [Google Scholar] [Crossref]
- A Labedz-Maslowska, N Bryniarska, A Kubiak, T Kaczmarzyk, M Sekula-Stryjewska, S Noga. Multilineage Differentiation Potential of Human Dental Pulp Stem Cells-Impact of 3D and Hypoxic Environment on Osteogenesis In Vitro. Int J Mol Sci 2020. [Google Scholar] [Crossref]
- J Qian, T Chen, W Lu, S Wu, J Zhu. Breast milk macro- and micronutrient composition in lactating mothers from suburban and urban Shanghai. J Paediatr Child Health 2010. [Google Scholar] [Crossref]
- Abd Allah, H Somia, Sally M Shalaby, Amal S El-Shal, El Nabtety, M Sameh, Tarek; Abd Khamis, El Rhman, A Shimaa, Mahmoud A Ghareb, Kelani, M Hesham. . Int Union Biochem Molecular Biology Life 2016. [Google Scholar] [Crossref]
- S Patki, S Kadam, V Chandra, R Bhonde. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell 2010. [Google Scholar] [Crossref]
- Y Sakaguchi, I Sekiya, K Yagishita, T Muneta. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 2005. [Google Scholar] [Crossref]
- Z Si, X Wang, C Sun, Y Kang, J Xu, X Wang. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed Pharmacother 2019. [Google Scholar] [Crossref]
- R Yang, H Gao, L Chen, N Fang, H Chen, G Song. Effect of peripheral blood-derived mesenchymal stem cells on macrophage polarization and Th17/Treg balance in vitro. Regen Ther 2020. [Google Scholar] [Crossref]
- W Guofeng, P Mengjie, W Xianghai, W Jinkun, C Shangtao, L Zhenlin. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci Rep 2015. [Google Scholar] [Crossref]
- PP Chong, L Selvaratnam, AA Abbas, T Kamarul. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 2012. [Google Scholar] [Crossref]
- G Wu, M Pan, J Wen, S Cao, Z Li, Y Li. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci Rep 2015. [Google Scholar] [Crossref]
- C De Bari, F Dell'Accio, J Vanlauwe, J Eyckmans, IM Khan, CW Archer. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum 2006. [Google Scholar] [Crossref]
- M Bozorgmehr, S Gurung, S Darzi, S Nikoo, S Kazemnejad, AH Zarnani. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front Cell Dev Biol 2020. [Google Scholar] [Crossref]
- Jieyuan ; Zhang, Guan, ; Junjie, Niu, ; Xin, Hu, ; Guowen, Guo, ; Shangchun, Li, ; Qing, Xie, ; Zongping, Zhang, ; Changqing, Yang Wang. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. Journal of Translational Medicine 2015. [Google Scholar]
- Introduction
- Immunosuppressive and Immunomodulatory Properties of MSCs
- Sources and their potentials
- Umbilical cord derived MSCs (UC-MSCs)
- Amniotic fluid derived MSCs
- Adipose tissue derived MSCs (ATMSCs)
- Dental pulp derived MSCs (DPSCs)
- Breast milk derived MSCs
- Synovium derived MSCs
- Peripheral blood derived mesenchymal stem cells (PBMSCs)
- Periosteum derived MSCs
- Menstrual fluid derived MSCs
- Induced pleuripotent MSCs
- Comparison of potentials of various sources of MSCs
- Conclusion
- Conflict of Interest
- Source of Funding
